Programmable Molecular Technology Initiative: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
| | | | ||
Current participants: | Current participants: | ||
{{project current participants}} | |||
Additional participants: | |||
{{project additional participants}} | |||
| | | | ||
Collaborators: | Collaborators: | ||
| Line 13: | Line 14: | ||
Previous participants: | Previous participants: | ||
{{project past participants}} | |||
* {{Emzo de los Santos}} | * {{Emzo de los Santos}} | ||
* {{Dan Siegal-Gaskins}}* | * {{Dan Siegal-Gaskins}}* | ||
Revision as of 02:41, 11 June 2016
The information on this page focuses primarily on the work involving my research group.
|
Current participants: Additional participants: |
Collaborators:
Previous participants:
|
* Partially supported
Objectives
Biological organisms depend on remarkable molecular machines whose function is encoded within the molecules themselves – nucleic acid and protein sequences programmed by evolution to catalyze reactions, synthesize molecules, haul cargo, regulate development, and defeat pathogens. The proposed Programmable Molecular Technology Initiative (PMTI) will extend and exploit principles for engineering these versatile biomolecules with the mission of pioneering high-impact technologies centered in three focus areas: molecular instruments for readout and regulation of cell state, programmable molecular logic for selectively treating diseased cells while leaving normal cells untouched, and efficient microbial synthesis of biofuels from non-food renewable resources.
- Efficient microbial synthesis of biofuels from non-food renewable resources: We seek to achieve dramatic reductions in the cost of producing biofuels using re-programmed metabolic pathways in microbes, supporting an industrial revolution in the production of liquid fuels from renewable non-food resources. The focus of our work is in the re-programmed yeast stress response pathway that tolerates toxins generated during biofuel synthesis.
- Principles and foundations for programming molecular function: We seek to establish the underpinnings for future generations of programmable molecular technologies, including (1) languages, simulators and compilers for automated analysis and design of nucleic acid systems, (2) programmable self-organization of active molecular structures and (3) feedback control mechanisms for programming developmental patterning.
References
- System-level studies of a cell-free transcription-translation platform for metabolic engineering. Yong Y. Wu, Hirokazu Sato, Hongjun Huang, Stephanie J. Culler, Julia Khandurina, Harish Nagarajan, Tae Hoon Yang, Stephen Van Dien, Richard M. Murray. bioRxiv Technical Report.
- Design Space Exploration of the Violacein Pathway in Escherichia coli Based Cell-Free System. Phuc H.B. Nguyen, Yong Wu, Shaobin Guo, Richard M Murray. Submitted, 2016 Synthetic Biology: Engineering, Evolution and Design (SEED) Conference.
- Rapid in vitro engineering of 16 two-input logic gates. Clarmyra A. Hayes, Emmanuel L.C. de los Santos, Enoch Yeung, Sean R. Sanchez, Seung Y. Lee, and Richard M. Murray. Presented, 2016 Winter q-Bio.
- Prototyping 1,4-butanediol (BDO) biosynthesis pathway in a cell-free transcription-translation (TX-TL) system. Yong Y Wu, Stephanie Culler, Julia Khandurina, Stephen Van Dien, Richard M Murray. Presented at Synthetic Biology: Engineering, Evolution and Design (SEED), 10-13 June 2015.
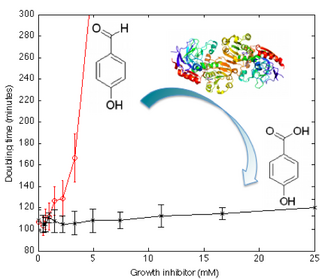
- Engineering Transcriptional Regulator Effector Specificity using Computational Design and In Vitro Rapid Prototyping: Developing a Vanillin Sensor. Emmanuel Lorenzo Cornejo de los Santos, Joseph T Meyerowitz, Stephen L Mayo, Richard M Murray. ACS Synthetic Biology, 5(4):287–295, 2016..
- Engineering Transcriptional Regulator Effector Specificity Through Rational Design and Rapid Prototyping. Emmanuel L.C. de los Santos, Joseph T. Meyerowitz, Stephen L. Mayo, and Richard M. Murray. Presented at Synthetic Biology: Engineering, Evolution and Design (SEED), 14-18 July 2014.
- Resource usage and gene circuit performance characterization in a cell-free âbreadboardâ. Dan Siegal-Gaskins, Zoltan A. Tuza, Jongmin Kim, Vincent Noireaux, Richard M. Murray. ACS Synthetic Biology, 3(6):416–425, 2014.
- Biomolecular resource utilization in elementary cell-free gene circuits, D. Siegal-Gaskins, V. Noireaux, and R. M. Murray. American Control Conference (ACC), 2013.
This research is funded by the Gordon and Betty Moore Foundation through Grant GBMF2809 to the Caltech Programmable Molecular Technology Initiative.
|
|
|