TX-TL projects, 2015-16
|
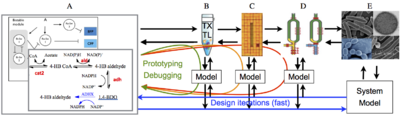
| Overview of the cell-free expression breadboard process. |
The overall goal of this research is to build a set of “biomolecular breadboards” to create a systematic, engineering-oriented approach to synthesizing biomolecular circuits that involves developing, modeling, and debugging a sequence of prototype devices, each at increasing levels of complexity and each allowing the incorporation of increasingly realistic operating environments for either in vitro or in vivo applications. We are adapting an existing cell-free toolbox developed at U. Minnesota to create a set of prototyping environments for testing biological circuits. A sequence of increasingly complex environments, ending in prokaryotic cells, is being used to demonstrate the ability to prototype circuits that function in vivo, with iteration in in vitro assays and models to streamline development of predictable, in vivo functionality.
A number of projects are available for 2015-16 for undergraduates interested in contributing to the research progress in this area. Possible projects include:
- Variability in circuit performance across extract preparation methods. We have seen many situations in which circuits work only in batches of extract produced with certain methods of lysis (bead beating, homogenization, french press, etc). There are no obvious patterns of what types of circuits work in what types of extract, and so there are a lot of interesting speculation about what is going on. Work in this area would require testing existing circuits in different extract batches and then debugging the circuits in the batches where they don't work correctly.
- Freeze-drying TX-TL. Currently, extract & buffer have to be stored at -80C, making transportation and distribution to non-science facilities difficult. One potential solution is to freeze-dry, or lyophilize, TX-TL, which may preserve activity at higher temperatures. Research will focus on determining optimal lyophilization & storage conditions for TX-TL, and may include trying vacuum sealing, stoppering chambers, or desiccant. Students will also test different types of circuits in TX-TL, to determine how lyophilization effects circuit function. This project may overlap with the Paper-based TX-TL project, as this research includes lyophilizing TX-TL onto paper.
- Paper-based TX-TL. Currently, TX-TL reactions are run either in plate wells or microcentrifuge tubes, with the fluorescent output of reactions measured in a plate reader. However, recently researchers in the Murray lab have found a way to measure the output of TX-TL reactions with a cellphone, if the TX-TL reactions are on paper. This project focuses on developing paper-based technology for TX-TL, with the aim of eventually being able to run all reactions on paper and read all outputs on a cellphone. Research will include designing paper reaction strips, testing different circuits on the strips, and modifying the cellphone to measure multiple fluorescent and luminescent reporter proteins. This project may overlap with the Freeze-drying TX-TL project, as it may involve lyophilizing TX-TL onto paper.
- Colorimetric reporter proteins. Currently, the outputs of TX-TL reactions are almost always fluorescent proteins, easy to express in TX-TL and commonly used in biology. However, special excitation-emission filters are needed to measure fluorescence, making it difficult to measure outside of a laboratory setting. Finding a TX-TL output that can be seen by the naked eye, eg. a colored protein or an enzyme-mediated color change, would help enable TX-TL use in the field. Research focuses on testing the functionality of different colorimetric reporters, the LacZ enzyme and varied LacZ substrates in TX-TL. This project may overlap with the Paper-based TX-TL project, as it may include testing successful colorimetric reporter proteins on paper.
- Improvements in preparation methods for TX-TL. Current preparation methods to make extract for circuit prototyping are low-yield (18 mL per batch). However, alternative preparation methods exist (45 mL per batch) which are significantly less labor-intensive, but are not optimized for circuit prototyping. Research on preparation methods will be conducted to increase yields but match cellular conditions more precisely.